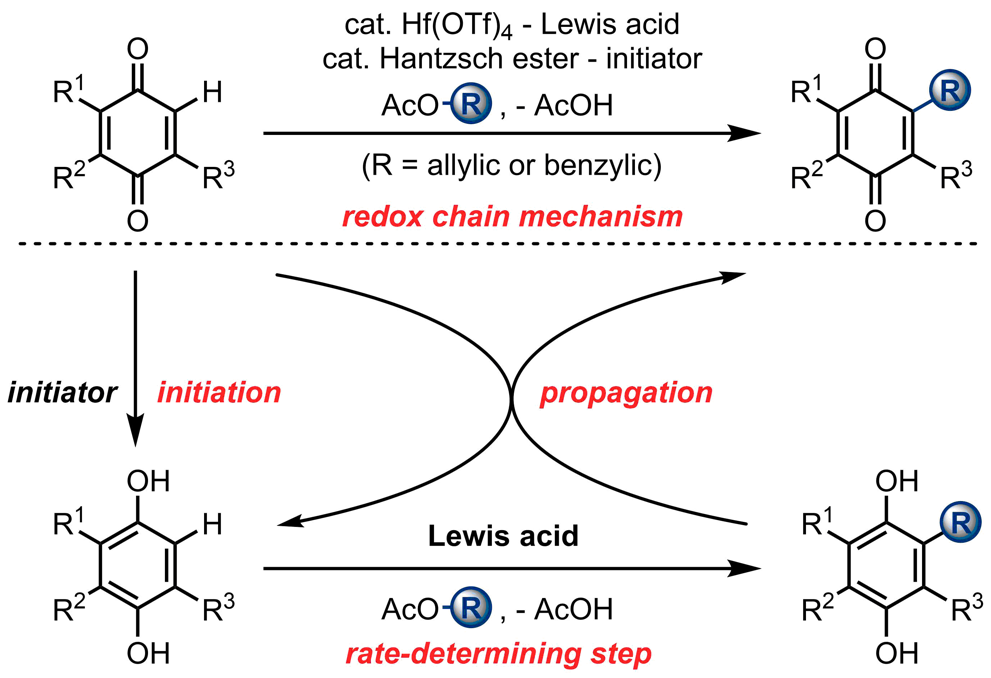
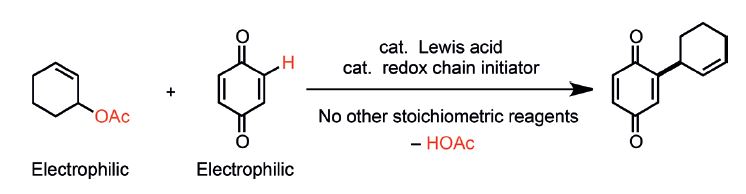
|  |
| Biography |
| 2002-2006 University of Science and Technology of China, B.S. (Advisor: Tianpa You) 2006-2011 The University of Chicago, USA, Ph.D. (Advisor: Hisashi Yamamoto) 2011-2014 Northwestern University, USA, Postdoctoral Fellow. (Advisor: Tobin J. Marks) 2015-2021 ShanghaiTech University, School of Physical Sciences and Technology, Assistant Professor, PI. 2021- Associate Professor, PI |
| Research Interests |
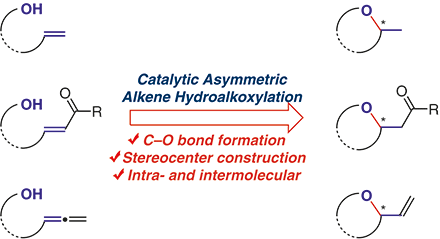
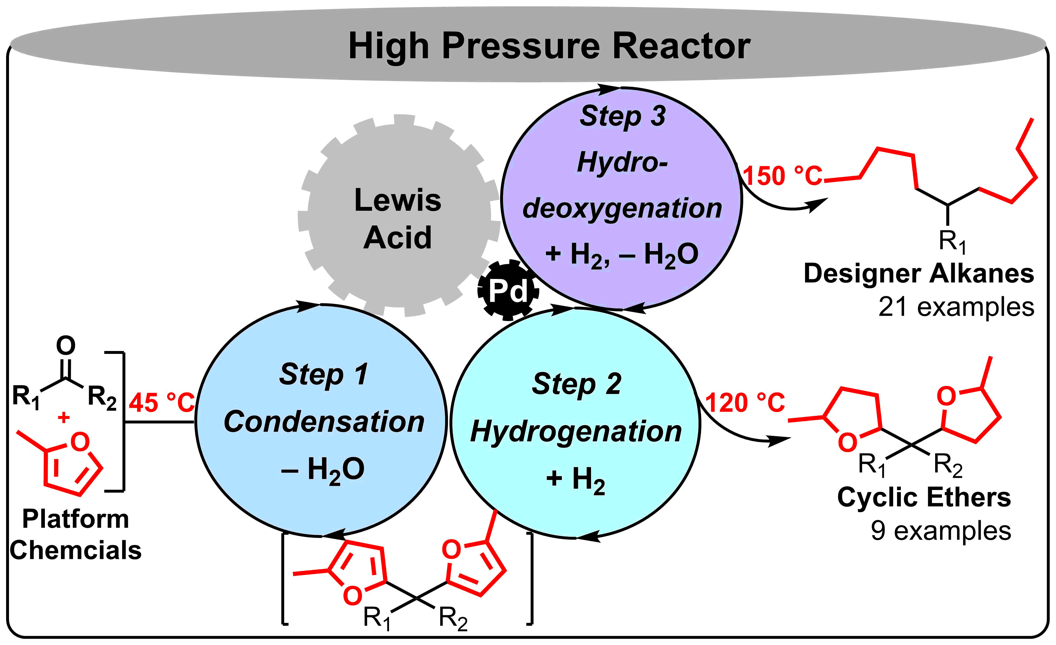
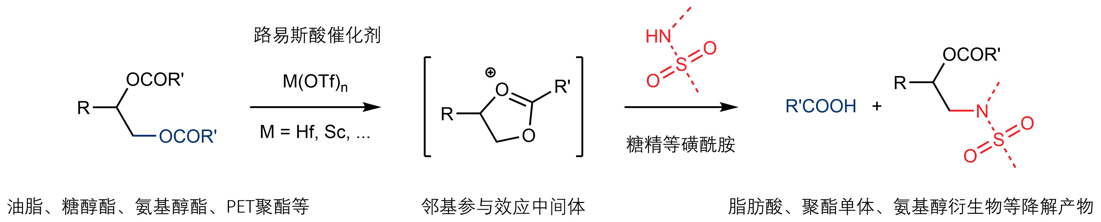
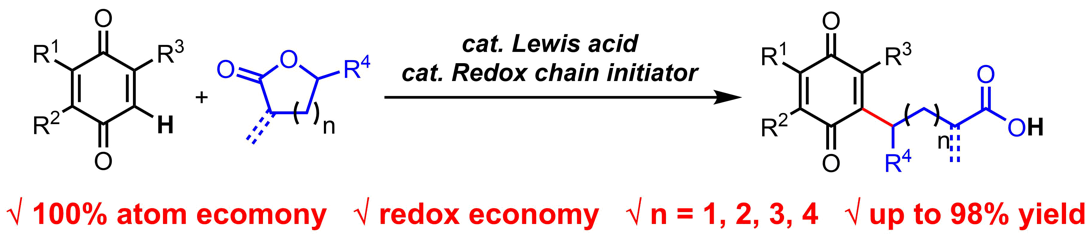
Catalysts and catalytic reactions have been pivotal players in modern chemical industry, which supplies the human society with almost all food, energy and materials in a general sense. They are also probably our most reliable ally when we are facing the unprecedented challenge of sustainable growth. For example, in order to solve problems in the emerging fields of carbon neutralization, renewable resources utilization and greener chemical processes, chemists’ ability to develop the most efficient yet sustainable catalysts and catalytic processes has never been more important. At ShanghaiTech, we will be developing novel strong Brønsted and Lewis acids as catalysts that can be used in highly efficient and sustainable chemical processes, especially those convert renewable biofeedstocks to useful energy and material products. We will also be investigating new chiral catalysts and catalytic asymmetric reactions that promote green syntheses of pharmaceutical ingredients. With our efforts we aim to accelerate the transition of chemical industry, particularly in China, from high emission and pollution to highly sustainable and green. |
| Publications |
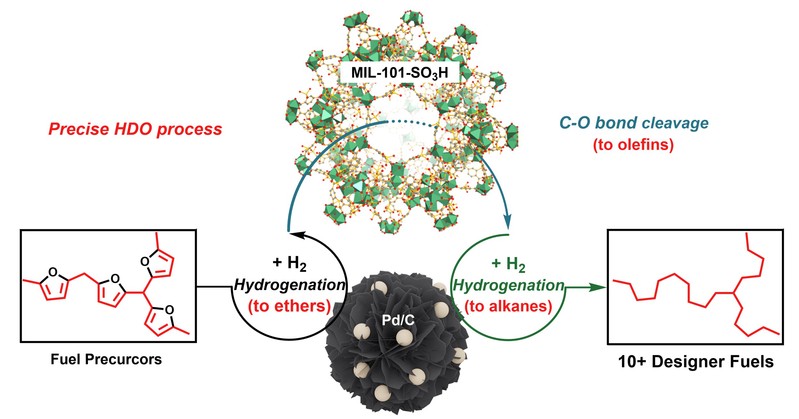
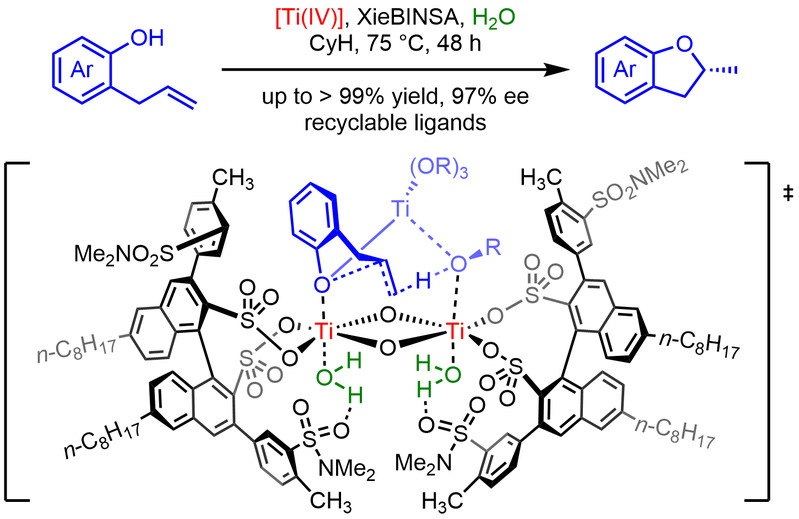
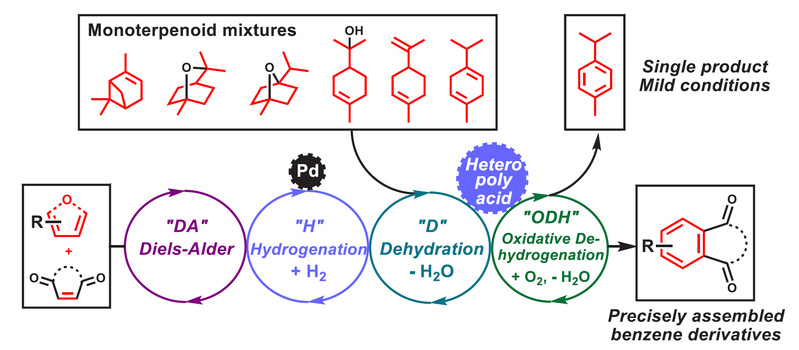
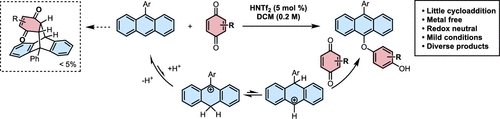
Independent Publications:
PhD & Postdoc publications: 1. Lohr, T. L.; Li, Z.; Marks, T. J.* Thermodynamic Strategies for C–O Bond Formation and Cleavage via Tandem Catalysis; Acc. Chem. Res., 2016, 49 (5), pp 824–834. 2. Lohr, T. L.; Li, Z.; Assary, R. S.; Curtiss, L. A.; Marks, T. J.* Mono- and tri-ester hydrogenolysis using tandem catalysis. Scope and mechanism; Energy Environ. Sci., 2016, 9 (2), pp 550–564. 3. Lohr, T. L.; Li, Z.; Marks, T. J.* Selective Ether/Ester C–O Cleavage of an Acetylated Lignin Model via Tandem Catalysis; ACS Catal., 2015, 5 (11), pp 7004–7007. 4. Lohr, T. L.†; Li, Z.†; Assary, R. S.; Curtiss, L. A.; Marks, T. J.* Thermodynamically Leveraged Tandem Catalysis for Ester RC(O)O–R′ Bond Hydrogenolysis. Scope and Mechanism; ACS Catal.,2015, 5 (6), pp 3675–3679. (Equal contribution) 5. Li, Z.; Assary, R. S.; Atesin, A. C.; Curtiss, L. A.; Marks, T. J.* Rapid Ether and Alcohol C-O Bond Hydrogenolysis Catalyzed by Tandem High-valent Metal Triflate + Supported Pd Catalysts; J. Am. Chem. Soc., 2014, 136, 104-107. 6. Assary, R. S.*; Atesin, A. C.; Li, Z.; Curtiss, L. A.*; Marks, T. J.* Reaction Pathways and Energetics of Etheric C–O Bond Cleavage Catalyzed by Lanthanide Triflates; ACS Catal., 2013, 3, 1908–1914. 7. Olivares-Romero, J. L.; Li, Z.; Yamamoto, H.* Catalytic Enantioselective Epoxidation of Tertiary Allylic and Homoallylic alcohols; J. Am. Chem. Soc., 2013, 135, 3411-3413. 8. Li, Z.; Yamamoto, H.* Hydroxamic Acids in Asymmetric Synthesis; Acc. Chem. Res.,2013,46, 506-518. 9. Olivares-Romero, J. L.; Li, Z.; Yamamoto, H.*; Hf(IV)-Catalyzed Enantioselective Epoxidation of NAlkenyl Sulfonamides and N-Tosyl Imines; J. Am. Chem. Soc., 2012, 134, 5440-5443. (Top 20 Most Read Articles of March 2012) 10. Li, Z.; Yamamoto, H.*; Zirconium(IV)- and Hafnium(IV)-Catalyzed Highly Enantioselective Epoxidation of Homoallylic and Bishomoallylic Alcohols; J. Am. Chem. Soc. 2010, 132, 7878-7880. 11. Li, Z.; Zhang, W.; Yamamoto, H.*; Vanadium-Catalyzed Enantioselective Desymmetrization of mesoSecondary Allylic Alcohols and Homoallylic Alcohols; Angew. Chem. Int. Ed., 2008, 47, 7520-7522; Angew. Chem., 2008,120, 7630-7632. |